Stopping the Spread of Coronavirus
About VeCoTek©
VeCoTek is a solution to coat surfaces to give them antibacterial and antiviral protection.

What Is:
VeCoTek®
VeCoTeK® Coating effectively interrupts the growth of microbes. The Anti-microbial and Anti-viral efficacy will last as long as coating remains on the surface. Scratches and surface wear do not adversely affect the coating’s efficacy.
VeCoTeK Main Characteristics:
- Safe
- Easy to use
- Cost effective
- Easy to manufacture
- Long lasting on surfaces
- Powerful antiviral anti-microbial properties
VeCoTeK® formulation can be adjusted to respond to different market needs.
VeCoTek Technology Advantages
Long Life
Antiviral / Antimicrobial Protection
Provides up to 99.99% protection of microbes
Environmentally Friendly
From natural source
Durability
in all seasons of the year with Consistent Performance
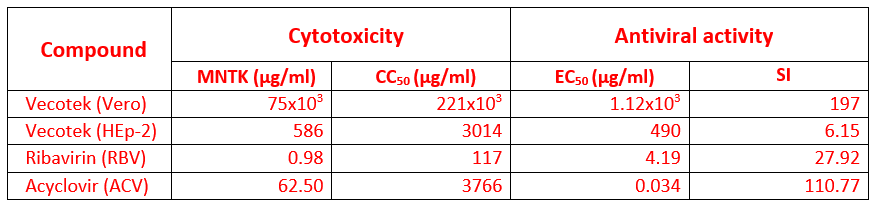
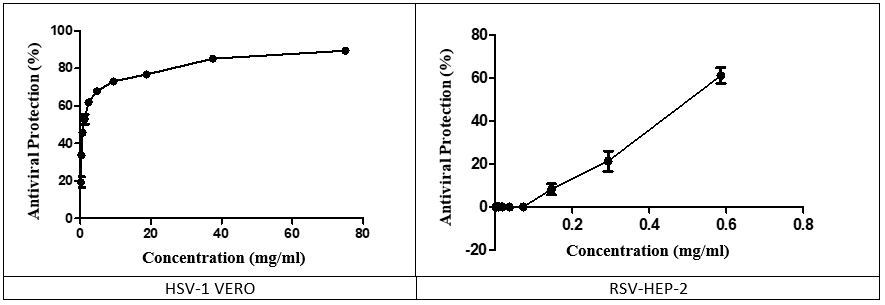
Antiviral Effects of VeCoTek.
Some compounds may interact virucidally with virus particles resulting in permanent inactivation of viral infectivity, with or without breaking down of virions. Vero (ATCC® CCL-81, African green monkey kidney epithelial cell) and HEp-2 (HEp-2 (ATCC® CCL-23, human epidermoid larynx carcinoma cell line) cells were used to determine the antiviral effect of the samples on HSV-1 (Herpes simplex virus, double-stranded DNA virus) and RSV (Respiratory syncytial virus, nonsegmented negative-strand RNA virus), respectively.
Cytotoxicity tests were examined separately with the XTT-based cell proliferation kit in accordance with the manufacturer’s instructions.

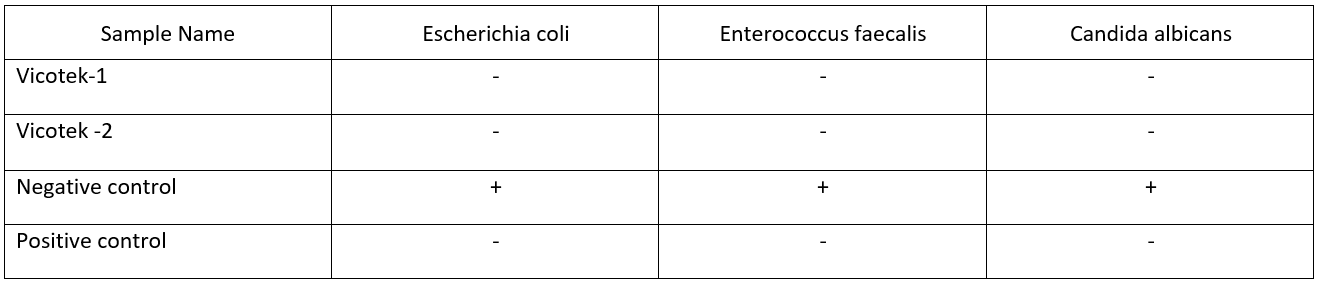
Antibacterial effects of VeCoTek
VeCoTek antibacterial effectiveness was determined by using the disk diffusion method according to the Clinical and Laboratory Standards Institute.
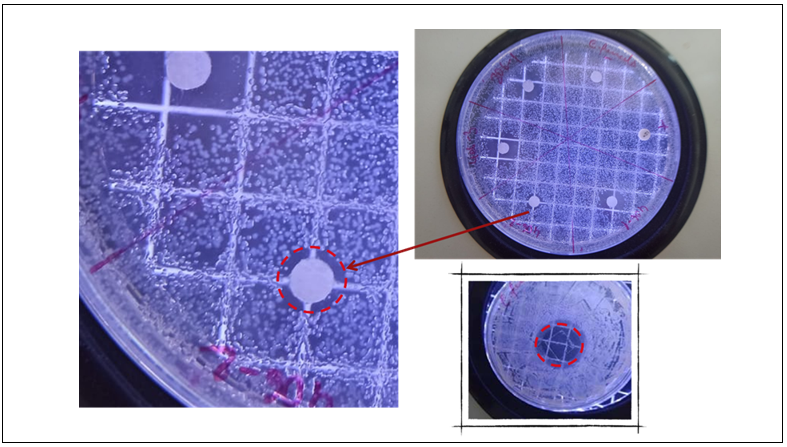
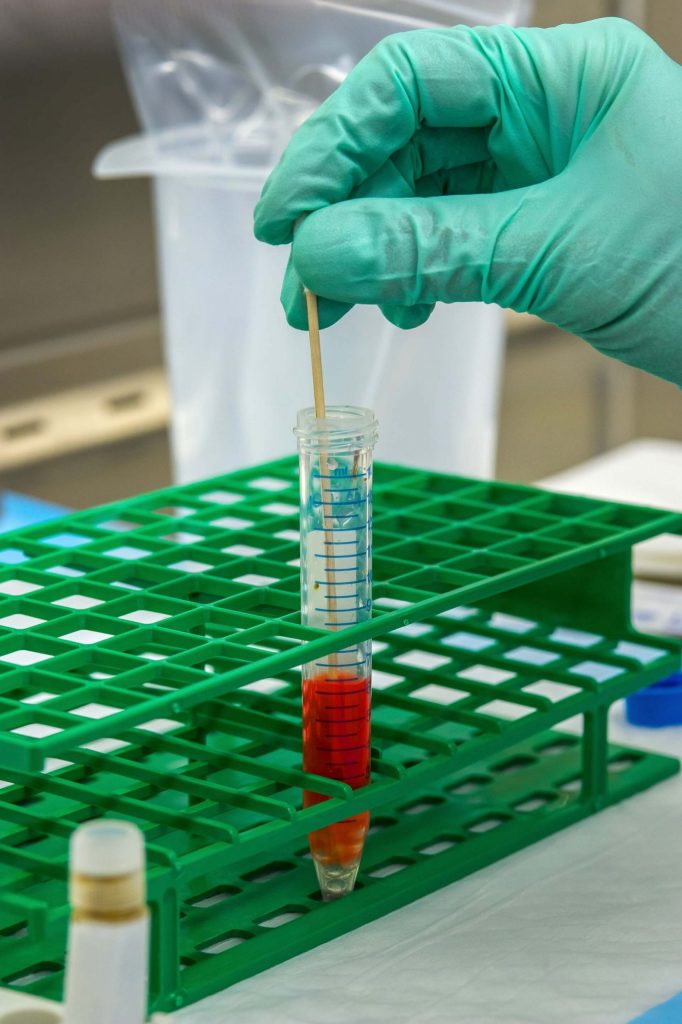
We also tested VeCoTek antibacterial effectiveness with a second method in different concentrations. In this method, VeCoTek was mixed with microorganism cultures with different ratios and tested in McFarland standards settings. After 24 h of treatment time, the sample was taken and transferred to Nutrient agar medium and incubated at 24 h for bacteria and 48 h for the yeast. The test microorganisms were Escherichia coli (gram negative), Enterococcus faecalis (gram positive), Candida albicans (yeast). Negative and positive control tests were performed under the same conditions. Microbial growth was measured via enumeration of viable cells on agar plates, over a period of 24 h for bacteria and 48 h for the yeast, at the appropriate temperatures. Concentration of sample did lead to significant increase in antimicrobial activity and we didn’t observe any bacterial/yeast growth on test. All samples showed same behavior with positive control tests which used antibiotic disks.